HELP PATIENTS START AND STAY ON TREATMENT WITH A FIRDAPSE TITRATION SCHEDULE

- FIRDAPSE comes in 10 mg scored tablets that can be split as needed2
- 60 mg was the average total daily dose of FIRDAPSE taken by adults in clinical trials36
- 100 mg is the total approved maximum daily dose—20 mg is the total maximum single dose2
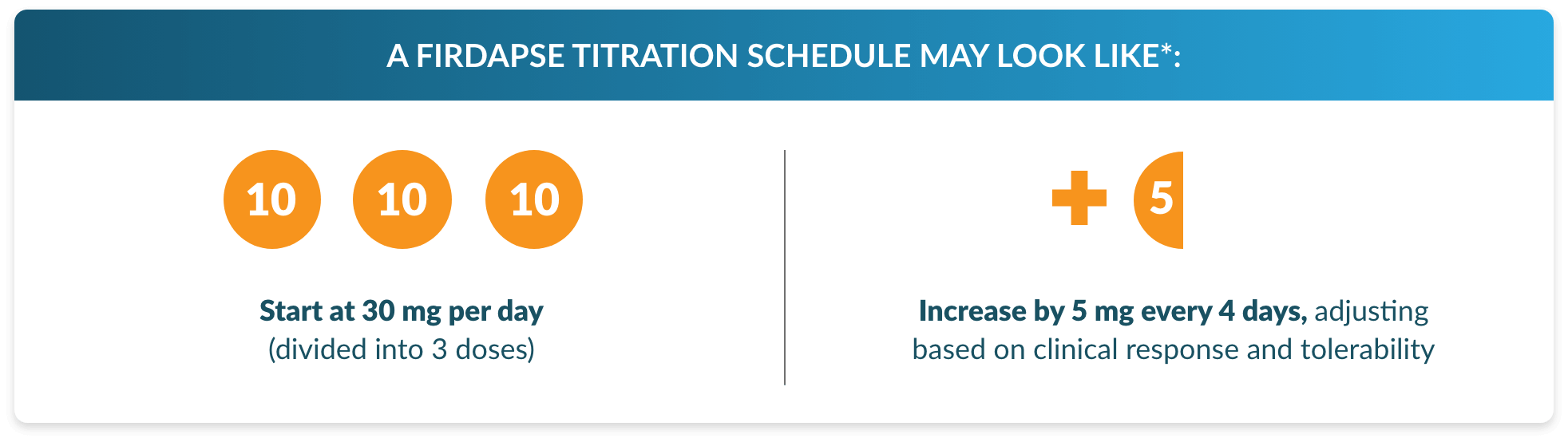
Help your patients reach their optimal dose
Titration is an important step for new patients who are just getting started on FIRDAPSE and have not taken
amifampridine before. By guiding your patients through the titration process, you can help them find their
optimal dose, which is key to:
- Maximizing symptom relief
- Minimizing tolerability issues, including paresthesia
FLEXIBLE OPTIMAL DOSING
LEMS is a progressive disease, and the dose of FIRDAPSE can change over time to address patients’ symptoms.
ENSURE YOUR PATIENTS WITH LEMS ARE SUFFICIENTLY TREATED TO MAXIMIZE NEUROMUSCULAR EFFICACY
This video will provide an overview of recommended FIRDAPSE dosing information, including example starting dosages, titration protocols, and a review of updated daily maximum dosages for both adults and pediatric patients with LEMS.

GET YOUR PATIENTS STARTED ON FIRDAPSE
Use the Enrollment Form to indicate a titration schedule and enroll your patient in Catalyst Pathways®.
For questions about enrollment, contact a FIRDAPSE Representative at
1-833-4-CATALYST (1-833-422-8259).
ADDITIONAL DOSING INFORMATION
- FIRDAPSE tablets can be taken with or without food2
- Scored tablets can be split with a pill splitter as needed2
- NOTE: If you have dosing adjustments <5 mg or have patients who have trouble swallowing a tablet, a suspension can be prepared. Refer to the Medication Guide for instructions on suspension preparation.2
- If a dose is missed, patients should not take double or extra doses2
- Patients newly treated with amifampridine should be advised that based on clinical trial observations, they may experience transient paresthesia, but this side effect is expected to diminish with continued treatment2,33
HOW FIRDAPSE IS SUPPLIED
FIRDAPSE is dispensed in both bottles and child-resistant blister packs, making it easy for you and your patients to choose the best option for them.
Bottles2
- Containing 60 or 240 tablets
Blister packs2
- Individual pack containing 10 tablets
- Carton containing 12 packs (120 tablets total)
Do you have a patient taking FIRDAPSE who is pregnant?
