FIRDAPSE IS THE RECOMMENDED FIRST-LINE TREATMENT FOR LAMBERT-EATON MYASTHENIC SYNDROME (LEMS)1
The only FDA-approved LEMS treatment for adults and pediatric patients 6 years of age and older1,2
A UNIQUE COMPOUND
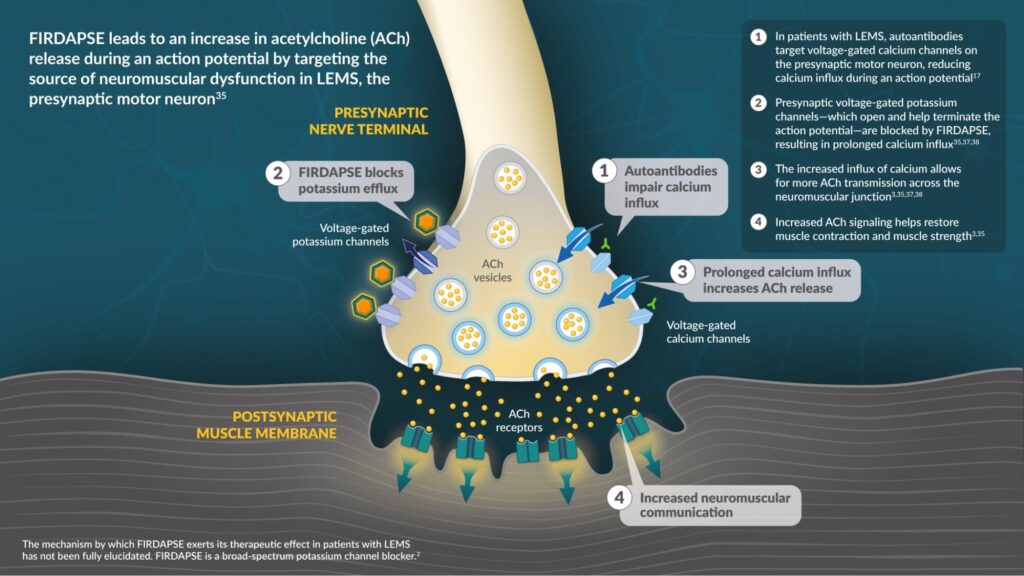
FIRDAPSE was the first FDA-approved therapy containing amifampridine, a voltage-gated potassium channel blocker that targets the presynaptic nerve terminal of neuromuscular junctions.33-35*
(3,4-diaminopyridine phosphate)
EXTENSIVELY TESTED
FIRDAPSE has been tested in more than 70 clinical and nonclinical studies, including two positive Phase 3 studies, over a 9‑year period.2,36
In clinical trials, FIRDAPSE demonstrated clinically meaningful preservation in muscle strength and patient satisfaction2-4:
(3,4-diaminopyridine phosphate)
CHANGING LIVES
FIRDAPSE is a broad-spectrum potassium channel blocker.2